In october 1999 I prepared this web version of my thesis from 1987. Only a few changes has been made, a few figures are now in colour and they are clickable to view in larger magnification. The text that were written in Word version 1.0 has been copied from the original diskettes. This document as a pdf file.
ACTA UNIVERSITATIS UPSALIENSIS
Comprehensive Summaries of Uppsala Dissertations from
the Faculty of Science
88
Rooting of Pinus sylvestris and Pinus contorta hypocotyl cuttings in vitro
by
Roland Grönroos

UPPSALA 1987
| 1 |
Doctoral dissertation to be publicly examined in the Botanical Auditorium, Uppsala University, on May 25, 1987, at 10.00 a.m., for the degree of Doctor of Philosophy.
Abstract
Direct rooting and rooting via a wound tissue in Pinus sylvestris L. and Pinus contorta Dougl. ex Loud. hypocotyl cuttings in vitro were studied. Direct rooting usually occurred within three weeks after cutting. Three to four weeks after cutting, roots developed either directly on the hypocotyl or via a wound tissue. Later than one month after cutting, rooting took place predominantly via a wound tissue. Anatomical studies showed that certain tissues in the hypocotyl could either form wound tissue or roots. Cuttings not treated with auxin developed a wound tissue from which roots eventually emerged. Wound tissues were usually fairly organized structures consisting of a central tracheid nest surrounded by meristematic tissue. Auxin treatment was necessary for direct rooting in vitro. When the cuttings were treated with auxin (IAA or IBA), roots usually developed outside resin ducts, in vertical rows along the length of the hypocotyl. Optimal auxin treatments for direct rooting of Pinus sylvestris and Pinus contorta were defined in terms of the time course for development of roots and the yield of rooted cuttings within one month. The optimal auxin treatments for Pinus contorta gave rooting percentages of more than 80% within 19 days and half of the cuttings which possessed roots after one month had acquired them within 14 days. For Pinus sylvestris, the optimal auxin treatments gave a lower yield (45%) of directly rooted cuttings.
Uptake studies with tritium-labeled IAA showed that the cuttings had a passive mode of IAA uptake. The optimal IAA treatment for direct rooting of Pinus contorta (6 h with 5.71 mM) resulted in a mean IAA concentration (5.55 µmol/g fresh weight) in the basal 3 mm of the hypocotyl that was similar to that in the treatment solution. Eighteen hours after transfer to new culture medium, the IAA concentration was 1.4 and 2.0 µmol/g fresh weight respectively in the upper and basal parts of the hypocotyl, which was where root initiation took place. Differences between individual cuttings in auxin uptake could not explain the poor direct rooting of Pinus sylvestris hypocotyl cuttings cultured in vitro.
In vitro culture inhibited root elongation growth. However, after transfer of rooted cuttings from in vitro to hydroponic culture, root elongation growth increased markedly.
Additional keywords - Adventitious, activated charcoal, anatomy, AOPP, auxin, bud, conifer, IAA, IBA, Lodgepole pine, mitosis, PAL, peroxidase, phenylalanine ammonia-lyase, root, Scots pine, seedling, tracheid, wound tissue.
Roland Grönroos, Department of Physiological Botany, Uppsala University, Box 540, S-751 21 Uppsala, Sweden.
ISSN 0282-7468
ISBN 91-554-2056-7
Uppsala 1987
© Roland Grönroos 1987
| 2 |
Preface
This thesis is a summary of studies related to rooting of Pinus hypocotyl cuttings in vitro. The studies are presented in the communications listed below and some previously unpublished results are also included. The communications are referred to by Roman numerals. The investigations have been carried out at the Department of Physiological Botany, Uppsala University, Sweden. Reprints were made with permission from the journals.
I. Grönroos, R. & von Arnold, S. 1985. Initiation and development of wound tissue and roots on hypocotyl cuttings of Pinus sylvestris in vitro. - Physiol. Plantarum 64: 393-401.
II. Grönroos, R. & von Arnold, S. 1987. Initiation of roots on hypocotyl cuttings of Pinus contorta in vitro. - Physiol. Plantarum 69: 227-236.
III. Grönroos, R. & von Arnold, S. 1987. Initiation of roots on hypocotyl cuttings of Pinus sylvestris, with emphasis on direct rooting, root elongation growth and auxin uptake. - Physiol. Plantarum. (submitted).
IV. Grönroos, R. & von Arnold, S. 1987. Direct rooting of hypocotyl cuttings of Pinus contorta in vitro with emphasis on IAA treatment, uptake and distribution. - Physiol. Plantarum. (submitted).
V. von Arnold, S. & Grönroos, R. 1986. Anatomical changes and peroxidase activity after cytokinin treatments inducing adventitious bud formation on embryos of Picea abies. - Bot. Gaz. 147: 425-431.
Distinction of contributions according to Swedish statute-book (Svensk författningssamling 1977:263, 8 kap, 35): Roland Grönroos is responsible for all parts in I, II, III and IV. In V, Roland Grönroos is responsible for all parts concerning peroxidases.
| 3 |
| Contents | page |
| Abstract | 2 |
| Preface | 3 |
| Introduction | 3 |
| Background | 6 |
| Rooting of conifers | 6 |
| Selection of plant material | 6 |
| Auxin treatment and uptake | 7 |
| Inhibition of tracheid formation | 8 |
| Marker for root initiation | 9 |
| Results and discussion | 10 |
| Plant material and root formation (I, II, III and IV) | 10 |
| Hypocotyl anatomy (I and II) | 10 |
| Wound tissue formation and rooting via a wound tissue (I, II and III) | 11 |
| Auxin treatments (II, III and IV) | 12 |
| Initial anatomical events in direct rooting (II) | 13 |
| Anatomy and morphology of direct rooting (II and III) | 14 |
| Intermediate type of rooting (previously unpublished) | 14 |
| Root anatomy (II and III) | 15 |
| Auxin uptake (III and IV) | 15 |
| Comparison between in vitro and hydroponic culture (II and III) | 16 |
| Effect of activated charcoal (I) | 17 |
| Effects of AOPP on wound tissue formation and of light period on rooting of Pinus sylvestris hypocotyl cuttings (previously unpublished) | 17 |
| Marker system for adventitious bud initiation in Picea abies (V) | 19 |
| Conclusions and research suggestions | 19 |
| Acknowledgements | 20 |
| References | 22 |
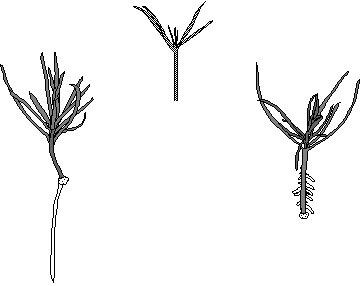
Cover. Hypocotyl cuttings of Pinus contorta cultured in vitro; top, immediately after preparation from a nineteen-day-old seedling; below left, two months after cutting, one root emerging from wound tissue; below right, one month after cutting and treatment with 5.71 mM IAA for 6 h, 23 roots emerging from the hypocotyl. From II, Fig. 1 and Fig. 14 and from IV, Fig. 2.
Abbreviations - AOPP, a-amino-oxy-ß-phenylpropionic acid; BA, N6-benzyladenine; IAA, 3-indolylacetic acid; IBA, 3-indolylbutyric acid; PAL, phenylalanine ammonia-lyase.
| 4 |
Introduction
Cloning or vegetative propagation of trees has been "a useful tool in traditional tree improvement", and it "promises to be the basis of a revolution in tree improvement" (Libby 1986). "The great promise of clones is for production forestry" (Libby 1986). However, if clonal forestry practice becomes more intensive and continues for several tree generations, the natural genetic base of the forests may deteriorate and could even be destroyed (Krugman 1986, Olsson 1985). "We are responsible for making sure that biotechnology poses no threat to the ecological systems we wish to manipulate as well as to maintain" (Krugman 1986).
Conifers may be vegetatively multiplied in several different ways, for example via grafting, cuttings, adventitious shoots or somatic embryos. For a review, see Dunstan and Thorpe (1986) or Thorpe and Biondi (1984). The two latter methods benefit from or require the use of in vitro techniques for large scale propagation. Commercial production of Pinus radiata by tissue culture methods with production levels of two million plants per year is under development by Tasman Forestry Limited in New Zealand (Gleed 1985). However, rooting of adventitious shoots (Aitken-Christie and Thorpe 1984) and root development on embryos (Hakman and von Arnold 1985) are often limiting steps. For example, only about 10% of vigorous adventitious shoots from embryos of Pinus sylvestris rooted spontaneously after about 10 months in culture (Shen and Arnold 1982) and about 10% of Picea abies somatic embryos developed roots (M. Becwar personal communication 1986). During the 6:th International Congress of Plant Tissue and Cell Culture, attention was called to the necessity to study root quality (Boulay 1986, Mott et al. 1986) since poor growth of in vitro multiplied conifers had been observed in the field. To sum up, there is a considerable demand for increased knowledge about rooting and root quality in conifers propagated in vitro. The aim of this project was to develop reproducible
| 5 |
culture methods for rooting of conifers, especially Pinus sylvestris, in vitro and to study root quality.
Background
Rooting of conifers
In general, rooting in conifers is a slow process. This is partly because cuttings of most conifers, especially Pinus, Abies and Picea, produce roots via a wound tissue (Satoo 1956). Nevertheless, direct rooting of cuttings, without formation of a wound tissue, may occur and is generally faster than rooting via a wound tissue. In Pinus banksiana (Montain et al. 1983) and in Cryptomeria japonica, Chamaecyparis pisifera, Thuja occidentalis and Pinus strobus (Satoo 1952, 1955), spontaneous direct rooting of cuttings has been reported and in Pinus radiata, direct rooting occurs after IBA treatment (Smith and Thorpe 1975a,b). Development of adventitious roots may also occur directly on the hypocotyl of intact plants of Cypressus sempervirens and Taxus baccata (Van Tieghem and Douliot 1888).
Selection of plant material Heterogeneity of the plant material, slow growth and labour intensive production of adventitious shoots in vitro, together with disappointing experiences with rooting, made adventitious shoots impractical for rooting studies. Therefore, a model system for rooting in vitro was looked for. A uniform and easily produced cutting material with documented rooting ability, suitable for in vitro culture, was required. Studies on rooting in the model system would yield knowledge that could later be used to develop rooting methods suitable for in vitro produced adventitious shoots of Pinus sylvestris. Pinus sylvestris has been considered a difficult to root species (Whitehill and Schwabe 1975). All examined adventitious roots of Pinus sylvestris developed from a wound tissue (Satoo 1956). However, during the last decade, several reports on successful rooting of juvenile Pinus sylvestris
| 6 |
material have been published (Eliasson and Strömquist 1981, Ernstsen and Hansen 1986, Hansen et al. 1978, Hansen and Ernstsen 1982, Phillion et al. 1983, Strömquist 1979, Strömquist and Hansen 1980, Whitehill & Schwabe 1975, Yli-Vakkuri and Pelkonen 1976). From these studies it seemed that the easiest material on which to induce roots was hypocotyl cuttings, i.e. the upper part of seedlings that were cut at the middle of the hypocotyl. Hypocotyl cuttings of Pinus sylvestris could be rooted without IBA treatment but a slightly faster rooting was obtained with IBA treatment (Strömquist and Hansen 1980). Hypocotyl cuttings of Pinus sylvestris were thus chosen as a model system for rooting in vitro.
During the initial studies on Pinus sylvestris (I), it became clear that rooting via a wound tissue was too slow and unpredictable. Therefore methods for direct rooting were required. Pinus contorta and Pinus radiata had been reported to be easy to root (Aldén and Chalupa 1984, Smith and Thorpe 1975a,b). An initial experiment showed that, after an IBA treatment, they could induce roots faster than Pinus sylvestris and anatomical studies showed that the roots developed directly on the hypocotyl. However, the large size of Pinus radiata hypocotyl cuttings made them impractical to work with in vitro. Pinus contorta was thus chosen as a model plant on which to study and improve the methods for direct rooting. Methods for direct rooting were developed and studied in Pinus contorta (II) and thereafter the knowledge about direct rooting was applied on Pinus sylvestris (III).
Auxin treatment and uptake
Plant growth regulators have become useful tools in agricultural and horticultural practices (Wareing and Phillips 1981). For example, the practical uses of auxins in flower induction, as herbicides and for rooting of stem cuttings have been found to have commercial value. Auxin treatment
| 7 |
and uptake were studied to find out whether direct rooting of Pinus contorta and Pinus sylvestris could be obtained by auxin treatments and, if so, how much auxin was necessary for direct rooting. Auxin treatment usually has a positive effect on rooting of conifers (Bowen et al. 1975, Hansen and Ernstsen 1982, Larsen and Dingle 1969, Patel and Thorpe 1984, Phillion et al. 1983, Whitehill and Schwabe 1975). In Pinus radiata, IBA was required for direct root initiation (Smith and Thorpe 1975b). Many auxin combinations, concentrations and treatment times have been tested and very different treatments which yield high rooting percentages have been found. For example, about 90% of Pinus sylvestris cuttings produced roots when treated either with 10 µM IBA for 6 weeks (Hansen and Ernstsen 1982) or with 20 mM IBA for 5 s (Phillion et al. 1983).
No studies concerning uptake of auxins to pine cuttings have been found. However, uptake of benzyladenine (BA) to Picea abies and Pinus sylvestris cuttings showed that BA uptake was passive (Vogelmann et al. 1984) and IAA uptake by Phaseolus aureus cuttings was proportional to the concentration of auxin in the treatment solution (Jarvis and Shaheed 1986). However, it should be remembered that factors other than auxin uptake may also limit rooting e.g. metabolism of auxin taken up (Haissig 1986), limited transport of auxin to the target cells (Jarvis 1986) or the target cells' ability to detect the applied auxin (Trewavas 1982).
Inhibition of tracheid formation
Due to the extensive differentiation of tracheids during wound tissue formation, it was reasoned that there might be a competition between rooting and tracheid differentiation. Therefore, an attempt to inhibit tracheid formation was made. The activity of the enzyme phenylalanine ammonia-lyase (PAL) has been correlated to xylogenesis (i.e. tracheid formation) and to the formation of vascular nodules (i.e. tracheid nests) in
| 8 |
callus (Haddon and Northcote 1976). PAL activity has also been suggested to be a rate-limiting step in lignification (Rubery and Fosket 1969). For a review on the role of PAL in plant development, see Jones (1984).
A potent inhibitor of PAL, a-amino-oxy-ß-phenylpropionic acid (AOPP), was described by Amrhein and Gödeke (1977). AOPP has been shown to be rapidly taken up by cells in suspension cultures and to inhibit PAL both in vivo and in extracts (Havir 1981). It was also possible to reverse AOPP inhibition of maize PAL but not of soybean PAL in extracts by dialysis (Havir 1981).
Marker for root and bud initiation
Since root initiation in wound tissue was difficult to predict, a marker for root initiation was desired to help in further studies on rooting via a wound tissue. There is substantial evidence that peroxidase activities in cuttings are related to rooting (Haissig 1986) and in Cynara scolymus, peroxidase activity could be used as a rooting marker in efforts to improve rooting (Moncousin and Gaspar 1983). A relationship between bud formation and peroxidase activity changes has also been shown (Legrand and Vasseur 1972, Nakanishi 1979, Thorpe et al. 1978, Mäder 1975, Thorpe and Gaspar 1978, Negrutiu et al. 1979, Kevers et al. 1981). Increase in peroxidase activity may favour bud initiation by changing the endogenous auxin-cytokinin ratio since peroxidases have auxin oxidase activity (Kevers et al. 1981). For an overview of plant peroxidases, see Gaspar et al. (1982) and Greppin et al. (1986). Since adventitious buds formed without callus formation on embryos of Picea abies and their development had been carefully characterized (von Arnold 1982; von Arnold and Eriksson 1985; V), isolated embryos of Picea abies were used for studying peroxidase activity as a marker system for differentiation in conifers. Variation in peroxidase activity during bud initiation has not previously been studied in conifers.
| 9 |
Results and discussion
Plant material and root formation (I, II, III and IV)
A uniform seedling material of Pinus sylvestris L. and Pinus contorta Dougl. ex Loud. was produced in vitro. After a germination and growth period of 2 to 4 weeks, cuttings were prepared (see cover). By that time, at least two of the cotyledons had been released from the seed coat. The cuttings were severed approximately at the middle of the hypocotyl, well above the transition zone between root and hypocotyl. Cuttings developed a wound tissue from which roots eventually emerged (see cover). Cuttings treated with auxin (IAA or IBA), developed roots directly on the hypocotyl (see cover).
Hypocotyl anatomy (I and II)
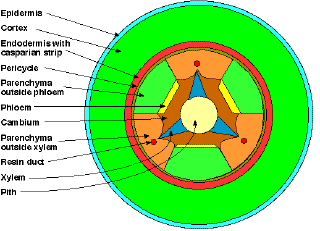
Seedlings of Pinus sylvestris and Pinus contorta had very similar hypocotyl anatomies (Fig. 1) (I, Fig. 2; II, Fig. 2). They both had a lignified epidermis with stomata, a cortex composed of chlorenchyma, an endodermis with casparian strip, a pericycle, a parenchyma layer, alternating xylem and phloem strands, cambium and a pith.
Fig. 1. Tissue map of the hypocotyl of Pinus contorta.
A resin duct is frequently formed centrifugally to the xylem.
From II, Fig. 2.
| 10 |
Wound tissue formation and rooting via a wound tissue (I, II and III)
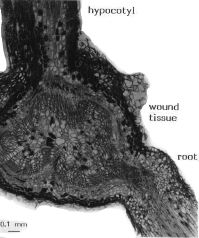
Cells which were cut by the knife during preparation of the cuttings died but adjacent intact cells usually survived. After six days these cells had started to enlarge and the cell division frequency in the basal part of the hypocotyl had increased, especially in the cortex, pericycle outside xylem, parenchyma outside xylem and cambium (II). Tracheids shorter than those normally found in the hypocotyl differentiated. After about two weeks, the epidermis and cortex split open (I, Figs 7-8). Usually an aggregate of short tracheids (tracheid nest) formed during the third week. A meristematic tissue surrounded the tracheid nest on all sides except the connection to the vascular system of the cutting (I, Figs 9-10). Cell divisions in the meristematic tissue increased the size of the wound tissue and after three months it consisted of a fairly organized woody structure with a diameter of 3-4 mm (Fig. 2) (I, Figs 15-16 and 19-20; II, Figs 14-15). This type of
| 11 |
wound tissue was commonly observed on cuttings cultured in solid medium. However, other types of wound tissue were also observed, some that almost entirely consisted of irregularly arranged short tracheids (I, Fig. 22) and others that were fairly callus like, with large parenchymatic cells (I, Fig. 23; II, Figs 12-13). The latter type was most commonly observed during culture in liquid medium.
After three weeks, some cuttings started to form roots from the wound tissue. About 40% of the cuttings were rooted within two months (I). Rooting continued for at least nine months (III). The age of the seedlings did not affect the rooting percentage or the number of roots per rooted cutting. Sometimes, only a few tracheids were present in the transition zone between root and wound tissue in Pinus contorta (Fig. 2). In Pinus sylvestris, the vascular connection between root and wound tissue was more sturdy than in Pinus contorta.
Auxin treatments (II, III and IV)
Auxin treatment was necessary for direct rooting in vitro. Optimal treatments of IBA and IAA for direct rooting were defined in terms of the time course for development of roots and the yield of rooted cuttings within one month. Treatments considered to be optimal for direct rooting of Pinus contorta gave rooting percentages of more than 80% within 19 days and half of the cuttings which possessed roots after one month had acquired them within 14 days (Fig. 3) (II). This type of rooting was obtained after
| Fig. 3. Time course for rooting of hypocotyl cuttings of Pinus contorta after various IBA-treatments. Optimal IBA treatment 80 µM IBA for 4 days (o). Suboptimal IBA treatment 80 µM IBA for 1 day (o). Superoptimal treatment 80 µM IBA for 8 days (o). Data are based on at least 27 cuttings per treatment. From II, Fig. 5. |
| 12 |
treatments with 80 µM IBA for 4 to 6 days, 1.25 to 5 mM IBA for 6 h or 5.71 mM IAA for 6 h (II and IV). Suboptimal treatments gave lower yields of rooted cuttings and superoptimal treatments resulted in delayed rooting or in partial necrosis of some cuttings (Fig. 3) (II and IV). Similarly, an optimal IBA treatment was defined for Pinus sylvestris. However, in Pinus sylvestris, the highest yield of rooted cuttings within three weeks (i.e. the period of direct rooting) was only 45% after an optimal IBA treatment (III). One explanation for the lower yield of rooted cuttings of Pinus sylvestris could be that high IBA concentrations and/or longer treatments, which might otherwise have improved rooting, harmed Pinus sylvestris cuttings. Direct rooting of Pinus sylvestris was 4-5 days slower than direct rooting of Pinus contorta, i.e. half of the cuttings which possessed roots after one month had acquired them after 18-19 days in Pinus sylvestris compared to 14 days in Pinus contorta. However, the time course for rooting of Pinus sylvestris after IBA treatment was considerably faster than previously described (Phillion et al. 1983, Satoo 1955, Strömquist and Hansen 1980). IAA functioned less well than IBA for direct rooting of Pinus sylvestris (III).
Initial anatomical events in direct rooting (II)
Six days after cutting, the cell division frequency in the basal 0.2 to 2.4 mm of the hypocotyl was much higher after an optimal IBA treatment (about 120 mitotic figures per mm) than in untreated cuttings (about 20 mitotic figures per mm). The highest mitotic activity was found in the parenchyma outside xylem. Root initials or root apical meristems in the hypocotyl could not be identified 6 days after cutting. Other tissues with high mitotic activities were the cortex, pericycle outside xylem and cambium. The mitotic activities in these tissues and in the parenchyma outside xylem were similar 0.2 to 2.4 mm from the cut surface of IBA treated cuttings (which developed roots directly on the hypocotyl) and 0 to 0.2 mm from the cut surface of non-treated cuttings (which developed
| 13 |
wound tissue). It was therefore concluded that, depending on the position and treatment, similar tissues may produce either wound tissue or roots.
Anatomy and morphology of direct rooting (II and III)
Roots usually formed in rows along the length of the hypocotyl, outside the resin ducts, which were parallel with and centrifugal to the primary xylem (Fig. 4) (III, Fig. 2). Occasionally, roots also formed in other places in the hypocotyl. Direct rooting usually occurred within three weeks after cutting. Three to four weeks after cutting, roots developed either directly on the hypocotyl or via a wound tissue. Later than one month after cutting, rooting took place predominantly via a wound tissue. However, when IBA treatment was delayed to one month after cutting, direct rooting could be obtained within three weeks from the treatment time(III).
Intermediate type of rooting (previously unpublished)
It might be necessary to discriminate an intermediate type of rooting from rooting via a wound tissue and direct rooting. The intermediate type of rooting is common in hydroponic culture (non-sterile conditions) and is characterized by roots that develop with almost the same time course as
| 14 |
direct rooting but at or very close to the cut surface. These roots seemed to have a larger diameter and grow faster than roots produced higher up on the hypocotyl. More research has to be done to characterize the intermediate type of rooting.
Root anatomy (II and III)
The anatomy of the adventitious root apex was similar to that previously described for conifers (II) (Esau 1960, Wilcox 1954). The number of xylem strands in adventitious roots of Pinus contorta was always two. In adventitious roots of Pinus sylvestris, two, three or four xylem strands were observed. Since lateral long roots of Pinus sylvestris were always diarch and primary roots were diarch, triarch or tetrarch depending on the distance from the hypocotyl (Aldrich-Blake 1930, Noelle 1910 as quoted by Hatch and Doak 1933), adventitious roots of Pinus sylvestris were similar, in this respect, to primary roots.
Auxin uptake (III and IV)
Uptake studies with a fixed amount of tritium-labeled IAA mixed with various amounts of unlabeled IAA showed that the uptake of radioactivity was independent of the amount of unlabeled IAA in the medium (III, IV). The uptake of tritium occurred mainly by uptake of the nutrient solution. It was therefore concluded that the cuttings had a passive mode of IAA uptake. Release of tritium and distribution to different parts of the cutting took place only during the first 18 h after tritium-IAA treatment. The concentrations of IAA in the cuttings and medium were calculated from tritium measurements without taking breakdown or metabolism of IAA into consideration. The optimal IAA treatment for direct rooting of Pinus contorta (6 h with 5.71 mM) resulted in a mean IAA concentration (5.55 µmol/g fresh weight) in the basal 3 mm of the hypocotyl that was similar to that in the treatment medium. Eighteen hours after transfer to new
| 15 |
culture medium, the IAA concentration was 0.7 µmol/g fresh weight in the whole cutting and 1.4 to 2.0 µmol/g fresh weight respectively in the upper and basal parts of the hypocotyl, which was where root initiation took place. However, it is not clear how much of the auxin found in the hypocotyl influences the tissue in which root initiation occurs. It is probable that most of the IAA taken up is initially located in the xylem and has to be transported to the root initiating tissues. Although cuttings of Pinus sylvestris were about three times heavier than cuttings of Pinus contorta, the concentration of exogenous IAA in the cuttings were similar after treatment with 1.43 mM IAA for 6 h. Treatment of Pinus sylvestris with 1.43 mM IAA for 24 h resulted in a concentration of 0.91 µmol/g fresh weight (III). This represented a 2000 to 20000 times higher internal IAA concentration than the normal endogenous concentration of IAA found in Pinus sylvestris needles (Sandberg et al. 1982). However, it was very close to the IAA concentration (0.96 µmol/g fresh weight) found after an optimal IAA treatment of Pinus contorta (5.71 mM for 6h) (IV). Furthermore, after these treatments, the range of IAA concentrations in cuttings of both species was similar. Yet only a few cuttings of Pinus sylvestris rooted, while 82% of Pinus contorta cuttings rooted within 3 weeks after treatment with IAA. Therefore, it was concluded that inability to take up auxin or variations in auxin uptake was not a probable reason for the difficulties in obtaining direct rooting of Pinus sylvestris cuttings in vitro.
Comparison between in vitro and hydroponic culture (II and III)
In this study, hydroponic culture always refers to culture under non-sterile conditions. The uptake of IAA was faster when the cuttings were treated in hydroponic culture compared to treatment in vitro (III). After IBA treatment, the time course for rooting of Pinus sylvestris was similar in hydroponic culture and in vitro (III). However, within three weeks after cutting, the yield of rooted cuttings was higher in hydroponic culture (III).
| 16 |
Although the number of roots per rooted cutting was higher when the cuttings were cultured in vitro (III), the most pronounced difference between in vitro and hydroponic culture was the rate of root elongation (II, III). Root elongation was inhibited by in vitro conditions. Several possible explanations for this inhibition were investigated but the reasons for the poor root elongation growth are still unclear (III). The only way to obtain satisfactory root elongation growth was to transfer the cuttings to hydroponic culture.
Effect of activated charcoal (I)
Addition of activated charcoal was tested, since it had been shown to improve rooting in monocotyledons (Fridborg and Eriksson 1975), dicotyledons (Eliasson 1981) and conifers (Patel and Thorpe 1984).
Addition of activated charcoal to agar solidified medium stimulated rooting via wound tissue. About 75% of the cuttings had developed roots after two months compared to about 40% without activated charcoal. However, the response to activated charcoal was not similar in all experiments. Sometimes wound tissue growth was stimulated instead of rooting and large wound tissues from which no roots developed formed in up to 95% of the cuttings. However, these large wound tissues supported growth so that the cuttings obtained similar fresh weights as root forming cuttings in vitro. How activated charcoal stimulates rooting is still unclear, although it might be by adsorbing growth regulators or inhibitors (Fridborg et al. 1978, Weatherhead et al. 1978, Tyagi et al. 1980).
Effects of AOPP on wound tissue formation and of light period on rooting of Pinus sylvestris hypocotyl cuttings (previously unpublished)
PAL activity in the basal six mm of the hypocotyl during wound tissue formation in cuttings increased to a maximum that was about four times higher than in control seedlings six days after cutting. Thereafter, the PAL
| 17 |
activity declined. Nine days after cutting, it was similar in cuttings and seedlings. PAL activity was measured as change in absorbance at 290 nm per time and fresh weight. Treatment of cuttings with 0.1 mM AOPP for 6 days resulted in complete inhibition of growth. Higher concentrations and/or longer treatment times (i.e. 0.1 mM for 9 days; 0.5 mM for 6 days or 12 days) resulted in necrosis. For lower concentrations and/or shorter treatment times (i.e. 0.006 mM for 6 h, 24 h, 3 days, 6 days and 9 days; 0.1 mM for 6 h, 24 h and 3 days), the time was recorded when 50% of the cuttings had a split epidermis (the first sign of wound tissue formation) (see I, Fig. 8). For all treatments tested, there was a delay in splitting of the epidermis compared to controls. The diameter of the wound tissue that was formed on cuttings treated with 0.006 mM AOPP for 9 days was 1.1±0.1 mm. On control cuttings, the wound tissue diameter was 1.6±0.1 mm (means ± 95% confidence limits). Three weeks after cutting, lignified short tracheids were found (after phloroglycin-hydrochloric acid staining) in cuttings that had been treated either with 0.006 mM AOPP for 9 days or with 0.1 mM AOPP for 24 h. From these experiments it was clear that PAL activity had a peak during wound tissue formation, that AOPP could be used to inhibit wound tissue formation and that the inhibition was probably reversible. However, no rooting was obtained after AOPP treatment.
The effect of different light periods on rooting (without auxin treatment) in agar-solidified medium was tested by first producing seedlings in 8, 16 and 24 h light periods and thereafter culturing cuttings from each of the light period treatments under all three light conditions. No significant differences in rooting were found between the treatments. The photoperiodic effects on rooting of non-woody cuttings were recently reviewed by Andersen (1986). He concluded that long days usually promoted rooting, but the effect was not spectacular.
| 18 |
Marker system for adventitious bud initiation in Picea abies (V)
Similar, rather large, changes in peroxidase activity were observed in embryos that developed adventitious buds and in control embryos that did not developed any adventitious buds. Therefore, total peroxidase activity was not useful as a marker for direct bud initiation. Large increases in peroxidase activity were found during root initiation. However, peroxidase studies were not continued due to the discouraging results on a marker for bud induction. Furthermore, changes in peroxidase isoenzyme patterns during rooting possibly occur after the induction phase (Jarvis 1986).
Conclusions and research suggestions
This project has shown that at least two different types of root formation, direct rooting and rooting via wound tissue, can be obtained in Pinus sylvestris and Pinus contorta. Initiation of roots occurred in meristematic tissues either outside the resin ducts (i.e. in tissues that eventually would have developed into cambium) or in meristematic tissue layers in the outer part of a wound tissue. In vitro culture was not inhibitory to root initiation but root elongation was severely inhibited.
During this project, some questions were formulated but remain unanswered. These questions are given below as suggestions for future research. They are divided into three main groups.
1. Characterization of adventitious roots. Are adventitious roots comparable to primary roots? How fit are the roots to support the needs of the shoot for uptake and transport of nutrients and water? What are the gravitropic and chemotropic responses of adventitious roots? Can adventitious roots cooperate with mycorrhiza? Will normal, faster or slower growth rates of the cuttings be obtained compared to seedlings? Will adventitious roots show normal lateral root formation and root dormancy?
| 19 |
2. Application of auxin treatments on rooting. Can direct rooting be obtained in cuttings from old trees and adventitious buds of pines? What happens to the auxin taken up and why do we need so much auxin in the cuttings?
3. Root elongation growth in vitro. How may satisfactory root elongation growth be obtained in vitro on embryos and shoots? May aeration or stirring of the nutrient solution improve direct rooting or root elongation in vitro?
Acknowledgements I wish to express my genuine gratitude to professor Tage Eriksson, Head of the Department of Physiological Botany, for introducing me to this field of research and also for his support, ideas and interest during my studies.
My enthusiastic, lively and energetic supervisor, Docent Sara von Arnold, deserves all appreciation. She has really been a treasure full of knowledge, good advice, inspiration and understanding. Many thanks Sara.
Thank you, Karin Bjelke and Ann-Charlotte Johansson for all excellent assistance with sowing, sectioning and peroxidase measurements.
Each and every one in the plant tissue culture group are thanked for stimulating discussions and for pleasant friendship, especially Dr. Peter Engström, Docent Gunnar Fridborg, Dr. Inger Hakman, Monika Ohlsson, and Docent Anita Wallin for critically reading and commenting the manuscript.
To all my friends in Liège, especially to Professor Thomas Gaspar who taught me so much about peroxidases and life in Liège, merci beaucoup.
I am much obliged to Docent Kenneth Söderhäll and Dr. Mauritz Ramstedt for valuable discussions during PAL, peroxidase and G-6-P dH measurements. The PAL activity measurements were carried out in cooperation with Pamela Ronald. Thank you Kenneth, Moje and Pamela.
Many thanks to Ulla-Britt Sahlström and Wivi Olsson for showing me sectioning and staining techniques, to Gerd Johansson for introducing me to the oxidizer, to Dr. Anders Claesson for demonstrating to me the first computer at the department, to Professor Torsten Ingestad and co-workers for letting me use their equipment for culture in running nutrient solution and to Erkki Mattson-Djos for showing me how to use the porometer.
The PAL inhibitor, AOPP, was synthesized by Klaus Serk-Hansen. I have several times consulted him for in chemical questions, and in the french language. Thank you Klaus.
Thank you, Dr. Per Häggblom for all advice concerning radioactivity methods.
| 20 |
I really appreciate the work of the technical and administrative staff at the department. Thank you, Hans Stenström, Sven-Olof Ekström, Sonja Forsgren, Anita Jansson, Kauko Piispanen and Roger Halmstad.
During my time as assistant at courses, Margareta Nylander was always very supportive. Thank you Margareta.
I am very grateful for the seeds of Pinus sylvestris and Pinus contorta that were kind gifts from Martin Werner, forest officer at The Institute of Forest Improvement, Ekebo, Svalöv, and seeds of Pinus radiata which were obtained from New Zealand as a gift from Ms. Jennifer Aitken-Christie.
I will always realize the worth of the inspiring discussions outside the lab with Thomas Eriksson.
I want to express my gratitude to my father Karl Grönroos, my mother Tyyne Grönroos and my brother Erik Grönroos for always supporting me in my decisions.
Finally, my bride-to-be, Lotta Bergman deserves the greatest recognition of all for checking the language, for questioning my ideas and for her friendship. Many thanks Lotta.
Financial support has been provided by Jacob Wallenberg Reseach Foundation (Stora Kopparberg), Bo Rydins Foundation for Scientific Research (Svenska Cellulosa Aktiebolaget, SCA), EEC commission, Liljewalchs travel grants, the Rector of Uppsala University (Wallenberg Foundation) and Cellulosaindustriens forskingsstiftelse 1959-års fond.
| 21 |
References
- Aitken-Christie, J. & Thorpe, T.A. 1984. Clonal propagation: Gymnosperms -
- In Cell Culture and Somatic Cell Genetics of Plants (I.K.Vasil, ed.),Vol 1, pp. 82-95. Academic Press, Inc. Orlando, FL. ISBN 0-12-715001-3.
- Aldén, T. & Chalupa, V. 1984. Vegetative propagation of lodgepole pine by
- organ cultures. - In Ecology and Management of Forest Biomass Production Systems (K.Perttu, ed.), pp. 285-290. Dept. Ecol. & Environ. Res., Swed. Univ. Agric. Sci. Rep. 15.
- Aldrich-Blake, R.N. 1930. Plasticity of root system of Corsican pine in
- early life. Oxford Forestry Memoirs. No. 12.
- Amrhein, N. & Gödeke, K.-H. 1977. -aminoxy- -phenylpropionic acid - a
- potent inhibitor of L-phenylalanine ammonia-lyase in vitro and in vivo. - Plant Science Letters. 8: 313-317.
- Andersen, A.S. 1986. Environmental influences on adventitious rooting in
- cuttings of non-woody species. - In New Root Formation in Plants and Cuttings (M.B.Jackson, ed.), pp. 223-253. Martinus Nijhoff Publishers, Dordrecht. ISBN 90-247-3260-3.
- Boulay, M. 1986. In vitro propagation of forest tree species. - In VI
- International Congress of Plant Tissue and Cell Culture, August 3-8, Abstracts. (D.A.Somers, B.G.Gengenbach, D.D.Biesboer, W.P.Hackett & C.E.Green, eds.) p. 9. University of Minnesota, Minneapolis. USA.
- Bowen, M.R., Howarth, J. & Longman, K.A. 1975. Effects of auxins and
- other factors on the rooting of Pinus contorta Dougl. cuttings. - Ann. Bot. 39: 647-656.
- Dunstan, D.I. & Thorpe, T.A. 1986. Regeneration in forest trees. - In Cell
- Culture and Somatic Cell Genetics of Plants (I.K.Vasil, ed.),Vol 3, pp. 223-241. Academic Press, Inc. Orlando, FL. ISBN 0-12-715003-X.
- Eliasson, L. 1981. Factors affecting the inhibitory effect of indolylacetic
- acid on root formation in pea cuttings. - Physiol. Plantarum 51: 23-26.
- Eliasson, L. & Strömquist, L.-H. 1981. Interaction between auxin and
- catechol in induction of adventitious roots. - In Symposium on Clonal Forestry. Swedish University of Agricultural Sciences Department of Forest Genetics, Uppsala, Research Notes 32, pp. 73-79. ISBN 91-576-0959-4.
- Ernstsen, A. & Hansen, J. 1986. Influence of gibberellic acid and stock
- plant irradiance on carbohydrate content and rooting in cuttings of Scots pine seedlings (Pinus sylvestris L.). - Tree Physiology 1: 115-125.
- Esau, K. 1960. Anatomy of seed plants. - John Wiley & Sons, Inc. New
- York, London, pp. 180-186.
- Fridborg, G. & Eriksson, T. 1975. Effects of activated charcoal on growth
- and morphogenesis in cell cultures. - Physiol. Plantarum 34: 306-308.
- Fridborg, G., Pedersén, M., Landström, L.-E. & Eriksson, T. 1978. The
- effect of activated charcoal on tissue cultures: Adsorption of
metabolites inhibiting morphogenesis. - Physiol. Plantarum 43: 104-106.22
- Gaspar, Th., Penel, Cl., Thorpe, T. & Greppin, H. 1982. Peroxidases 1970-
- 1980. - Université de Genève, Centre de Botanique, Genève.
- Gleed, J. 1985. The commercial production of radiata pine by tissue culture
- methods: some aspects and considerations. - In International Conifer Tissue Work Group. Third meeting 12 - 16 august 1985, Rotorua, New Zealand, Abstracts. p. 5.
- Greppin, H., Penel, C. & Gaspar, Th. 1986. Molecular and Physiological
- Aspects of Plant Peroxidases. - Université de Genève, Centre de Botanique, Genève. ISBN 2-88164-001-X.
- Haddon, L. & Northcote, D.H. 1976. Correlation of the induction of various
- enzymes concerned with phenylpropanoid and lignin synthesis during differentiation of bean callus (Phaseolus vulgaris L.). - Planta 128: 255-262.
- Haissig, B.E. 1986. Metabolic processes in adventitious rooting of cuttings. -
- In New Root Formation in Plants and Cuttings (M.B.Jackson, ed.), pp. 141-189. Martinus Nijhoff Publishers, Dordrecht. ISBN 90-247-3260-3.
- Hakman, I. & von Arnold, S. 1985. Plantlet regeneration through somatic
- embryogenesis in Picea abies (Norway spurce). - J. Plant. Physiol. 121: 149-158.
- Hansen, J., Strömquist, L.-H. & Ericsson, A. 1978. Influence of the
- irradiance on carbohydrate content and rooting of cuttings of pine seedlings (Pinus sylvestris L.). - Plant Physiol. 61: 975-979.
- Hansen, J. & Ernstsen, A. 1982. Seasonal changes in adventitious root
- formation in hypocotyl cuttings of Pinus sylvestris: Influence of photoperiod during stock plant growth and of indolebutyric acid treatment of cuttings. - Physiol. Plantarum 54: 99-106.
- Hatch, A.B. & Doak, K.D. 1933. Mycorrhizal and other features of the root
- systems of Pinus. - Jour. Arnold Arbor. 14: 85-99.
- Havir, E.A. 1981. Modification of L-phenylalanine ammonia-lyase in soybean
- cell suspension cultures by 2-aminooxyacetate and L-2-aminooxy-3-phenylpropionate. - Planta 152: 124-130.
- Jarvis, B.C. 1986. Endogenous control of adventitious rooting in non-woody
- cuttings. - In New Root Formation in Plants and Cuttings (M.B.Jackson, ed.), pp. 191-222. Martinus Nijhoff Publishers, Dordrecht. ISBN 90-247-3260-3.
- Jarvis, B.C. & Shaheed, A.I. 1986. Adventitious root formation in relation to
- the uptake and distribution of supplied auxin. - New Phytol. 103: 23-31.
- Jones, D.H. 1984. Phenylalanine ammonia-lyase regulation of its induction,
- and its role in plant development. - Phytochemistry 23: 1349-1359.
- Kevers, C., Coumans, M., De Greef, W., Jacobs, M. & Gaspar, T. 1981.
-
Organogenesis in habituated sugarbeet callus: auxin content and protectors, peroxidase pattern and inhibitors. - Z. Pflanzenphysiol. 101: 79-87.
23
- Krugman, S.L. 1986. The ethical question. - J. For. 84(1): 40-41.
- Larsen, F.E. & Dingle, R.W. 1969. Vegetative propagation of Lodgepole Pine
- (Pinus contorta Dougl.) from needle fascicles. - For. Sci. 15: 64-65.
- Legrand, B. & Vasseur J. 1972. Evolution de l'acide ribonucléique, des
- protéines et de l'activité peroxydasique au cours de la culture in vitro de fragments de feuilles d'endive (Cichorium intybus L., var. Witloof). - C.R. Acad. Sci. Paris, Sér. D., 275: 357-360.
- Libby, W.J. 1986. Clonal propagation. - J. For. 84(1): 37-42.
- Mäder, M. 1975. Änderung der Peroxidase Isoenzymmuster in Kalluskulturen
- in Abhängigkeit von der Differenzierung. - Planta Med. (Suppl.): 153-162.
- Moncousin, Ch. & Gaspar, Th. 1983. Peroxidase as a marker for rooting
- improvement of Cynara scolymus L. cultured in vitro. - Biochem. Physiol. Pflanzen 178: 263-271.
- Montain, C.R., Haissig, B.E. & Curtis, J.D. 1983. Initiation of adventitious
- root primordia in very young Pinus banksiana seedling cuttings. - Can. J. For. Res. 13: 191-195.
- Mott, R.L., Amerson, H.V. & Frampton, L.J. 1986. Clonal propagation of
- Pinus taeda L. from juvenile and mature stock plants. - In VI International Congress of Plant Tissue and Cell Culture, August 3-8, Abstracts. (D.A.Somers, B.G.Gengenbach, D.D.Biesboer, W.P.Hackett & C.E.Green, eds.) p. 10. University of Minnesota, Minneapolis. USA.
- Nakanishi, S. 1979. Peroxydases et bourgeonnement de fragments de racines
- d'Endive (Cichorium intybus L.). - C.R. Acad. Sci. Paris, Sér. D, 289: 695-698.
- Negrutiu, I., Jacobs M. & Gaspar T. 1979. Leaf formation and peroxidases
- from Arabidopsis callus. - Z. Pflanzenphysiol. 91: 119-126.
- Noelle, W. 1910. Studien zur vergleichen Anatomie und Morpologie der
- Koniferen wurzeln mit Rücksicht auf die Systematik. - Bot. Zeit. 68: 169-266.
- Olsson, R. 1985. Levande skog - skogsbruket i naturvårdsperspektiv. -
- Svenska naturskyddsföreningen, Stockholm. ISBN 91-558-7101-1.
- Patel, K.R. & Thorpe, T.A. 1984. In vitro differentiation of plantlets from
- embryonic explants of lodgepole pine (Pinus contorta Dougl. ex Loud.). - Plant Cell Tissue Organ Cult. 3: 131-142.
- Phillion, B.J., deWitt, J. & Bunting, W.R. 1983. Propagation of juvenile
- Scots Pine cuttings under a 24-hour photoperiod. - Tree Planters' Notes. 34: 39-42.
- Rubery, P.H. & Fosket, D.E. 1969. Changes in phenylalanine ammonia-lyase
- activity during xylem differentiation in Coleus and soybean. - Planta 87: 54-62.
- Sandberg, G., Odén, P-C. & Dunberg, A. 1982. Population variation and
- diurnal changes in the content of indole-3-acetic acid of pine
seedlings (Pinus sylvestris L.) grown in a controlled environment. - Physiol. Plantarum 54: 375-380.24
- Satoo, S. 1952. Origin and development of adventitious roots in seedling
- cuttings of conifers (1). - Bull. Tokyo Univ. For. 43: 59-81.
- Satoo, S. 1955. Origin and development of adventitious roots in seedling
- cuttings of conifers (2). - Bull. Tokyo Univ. For. 48: 115-128.
- Satoo, S. 1956. Anatomical studies on the rooting of cuttings in coniferous
- species. - Bull. Tokyo Univ. For. 51: 109-157.
- Shen, X.-H. & Arnold, S.von. 1982. In vitro formation of adventitious
- plantlets from embryos of Pinus sylvestris L. - Scientia Silvae Sinicae 18(4): 405-408.
- Smith, D.R. & Thorpe, T.A. 1975a. Root initiation in cuttings of Pinus
- radiata
seedlings. I. Developmental sequence. - J. Exp. Bot. 26: 184-192.- Smith, D.R. & Thorpe, T.A. 1975b. Root initiation in cuttings of Pinus
- radiata
seedlings. II. Growth regulator interactions. - J. Exp. Bot. 26: 193-202.- Strömquist, L.-H. 1979. Some aspects on rooting of Pinus sylvestris (L.)
- cuttings. - Ph. D. thesis, University of Umeå. ISBN 91-7174-041-4.
- Strömquist, L.-H. & Hansen, J. 1980. Effects of auxin and irradiance on the
- rooting of cuttings of Pinus sylvestris. - Physiol. Plantarum 49: 346-350.
- Thorpe, T.A. & Biondi, S. 1984. Conifers. - In Handbook of Plant Cell
- Culture vol. 2. Crop species (W.R. Sharp, D.A. Evans, P.V. Ammirato, Y. Yamada, eds.), pp. 435-470. Macmillian Publishing Company, New York. ISBN 0-02-949230-0.
- Thorpe, T. A. & Gaspar, T. 1978. Changes in isoperoxidases during shoot
- formation in tobacco callus. - In Vitro 14(6): 522-526.
- Thorpe, T. A., Tran Thanh Van, M. & Gaspar, T. 1978. Isoperoxidases in
- epidermal layers of tobacco and changes during organ formation in vitro. - Physiol. Plantarum 44: 388-394.
- Trewavas, A.J. 1982. Growth substance sensitivity: The limiting factor in
- plant development. - Physiol. Plantarum 55: 60-72.
- Tyagi, A.K., Rashid, A. & Maheswari, S.C. 1980. Enhancement of pollen
- embryo formation in Datura innoxia by charcoal. - Physiol. Plantarum 49: 296-298.
- Van Tieghem, M.Ph. & Douliot, H. 1888. Recherches comparatives sur
- Lórigine des membres endogènes dans les plantes vasculaires. - Ann. Sci. Nat. 7, Botanique 8. pp. 525-526.
- Vogelmann, T.C., Bornman, C.H. and Nissen, P. 1984. Uptake of
- benzyladenine in explants of Picea abies and Pinus sylvestris. - Physiol. Plantarum 61: 513-517.
25
- von Arnold, S. 1982. Factors influencing formation, development and rooting
- of adventitious shoots from embryos of Picea abies (L.) Karst. - Plant Sci. Lett. 27: 275-287.
- von Arnold, S. & Eriksson, T. 1985. Initial stages in the course of
- adventitious bud formation on embryos of Picea abies. - Physiol. Plantarum 64: 41-47.
- Wareing, P.F. & Phillips, I.D.J. 1981. Growth & Differentiation in Plants.
- 3rd edition. pp. 143-144. - Pergamon Press. Oxford. ISBN 0-08-026350-X.
- Weatherhead, M.A., Burdon, J. & Henshaw, G.G. 1978. Some effects of
- activated charcoal as an additive to plant tissue culture media. - Z. Pflanzenphysiol. 89: 141-147.
- Whitehill, S.J. & Schwabe, W.W. 1975. Vegetative propagation of Pinus
- sylvestris
. - Physiol. Plantarum 35: 66-71.- Wilcox, H. 1954. Primary organization of active and dormant roots of noble
- fir, Abies procera. - Am. J. Bot. 41: 812-821.
- Yli-Vakkuri, P. & Pelkonen, P. 1976. Rooting of Scots Pine needle fascicles
- with different growth substances and media. - Silva Fennica 10: 337-341.
| 26 |
ACTA UNIVERSITATIS UPSALIENSIS
Comprehensive Summaries of Uppsala Dissertations from
the Faculty of Science
Editor: The Dean of the Faculty of Science
A doctoral dissertation from the Faculty of Science, Uppsala University, is either a complete monograph or a joint summary of a number of papers. A few copies of these papers are kept together with their summary at major Swedish research libraries, while the summary alone is more widely spread over the world. The aim of the series Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science is to collect these summaries in order to give them a bibliographical and distributional platform. (Until July 1985 the series was named "Abstracts of Uppsala Dissertations from the Faculty of Science".)
Distributor:
Almqvist & Wiksell International
Stockholm, Sweden
ISSN 0282-7468
ISBN 91-554-2056-7
Updated Thursday, 07-Feb-2008 14:47 by Roland Grönroos
e-mail: rolandg@it.uu.se
Location: http://user.it.uu.se/~rolagron/rooting/avhandling/avhandling87.shtml